Acids
Acids taste sour – in fact, the German word for acid is sauer. Because acids can damage cells, our stomach needs a special lining to protect it from the hydrochloric acid used to digest our food. We are familiar with some acids – citrus fruits, tomatoes and vinegar are acidic.
Acids react with most metals including magnesium to create hydrogen gas and a salt – there are lots of different types of salts in chemistry. They also react with a group of substances called carbonates to produce carbon dioxide gas, salt and water. Learn about the reactions of calcium carbonate (like limestone) in this article.
Bases
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Bases feel slippery to touch. This is because they can change the structure of proteins. A strong base can cause severe chemical burns because it starts to damage the proteins in your skin. Basic substances are used in many cleaning products.
The simple chemistry
An acid is a substance that produces hydrogen (H+) ions when it is added to water. A hydrogen ion is just the proton and no electron. If we look at the formulas of different acids, we can see that they all contain at least one H (hydrogen) – for example:
- HCl – hydrochloric acid
- H2SO4 – sulfuric acid
- HNO3 – nitric acid.
When we put a molecule of acid into water, it breaks apart. The science term for this is that it dissociates. For example hydrochloric acid (HCl) dissociates into hydrogen ions (H+) and chloride anions (Cl-).
The chemical difference between acids and bases is that acids produce hydrogen ions and bases accept hydrogen ions.
A base is a substance that neutralises acids. When bases are added to water, they split to form hydroxide ions, written as OH-. We call a base that has been added to water an alkaline solution.
If we look at some formulas for bases, we can see that they all contain hydroxide (OH-) ions – for example:
- NaOH – sodium hydroxide (caustic soda)
- NH4OH – solution of ammonia in water
- Ca(OH)2 – calcium hydroxide (builders’ lime)
If an acid and a base are added together, they react to form water (H2O) and a salt. An example you might be familiar with is brushing your teeth. The acid created from the bacteria on your teeth reacts with the base in your toothpaste. This reaction is called neutralisation.
Identifying and measuring acids and bases
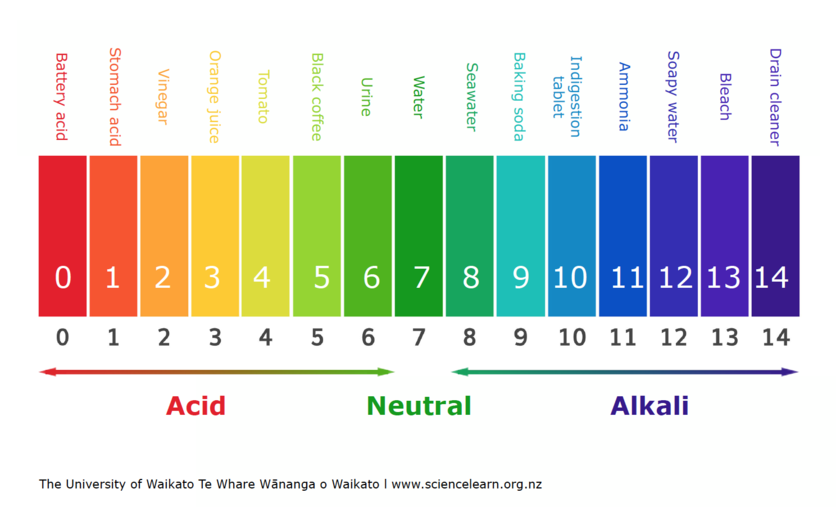
A pH meter measures how acidic or basic a solution is. When we test a substance with a pH meter, we get a number from 0–14. This is a pH scale, and it can be used to compare substances. It is important to know that this scale is logarithmic. This means that a decrease in the pH scale of 1 can result in an increase of 10 times the concentration of hydrogen ions.
Acids have a pH below 7. The more H+ ions, the more acidic it is and the lower the pH will be. Bases have a pH above 7. pH 7 is said to be neutral – this means there is a balance of H+ and OH- ions. Sometimes, the pH value can be less than 0 for very strong acids or greater than 14 for very strong bases.